CHART Techno Solutions Private Limited is a company incorporated in the city of Hyderabad, India.
CHART primarily focuses on Predictive and Deductive Data Analytics, Innovative Healthcare Oriented Solutions, Design and Development of Mobile Platforms and Developing Inventive Healthcare Technologies.
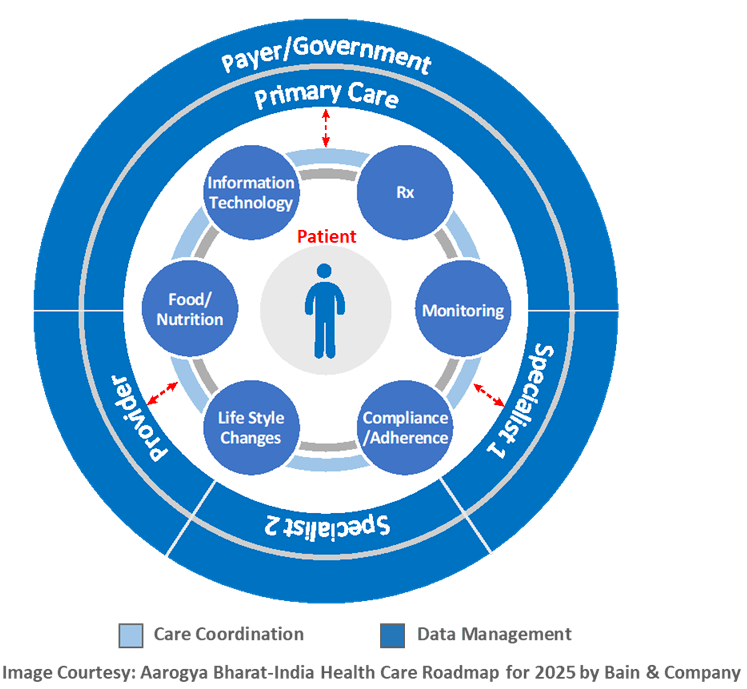
CHART has developed a comprehensive healthcare technology platform for Home and Onsite Healthcare to provide
- Instant Risk Analysis of Chronic Disease for Individuals across multiple therapeutic areas; including breast, oral, prostrate and cervical cancer.
- Online Deductive Analysis of Individuals on effect of Stress and other frequently occurring conditions such Compulsive Obsessive Disorder, Mania etc. that affects Productivity and Causes/Impacts Chronic Diseases
- Uniform Protocol and Process Oriented data acquisition Tool for Home and Assisted Living Healthcare Organizations
- Remote Monitoring of Rural Population and Elderly Citizen Living in Assisted Living Centers
- Automation and instant communications ( Alerts and Actions) to and from Medical professionals to ICUs and nurses
- Reduction of Total Cost of Healthcare for the Individuals
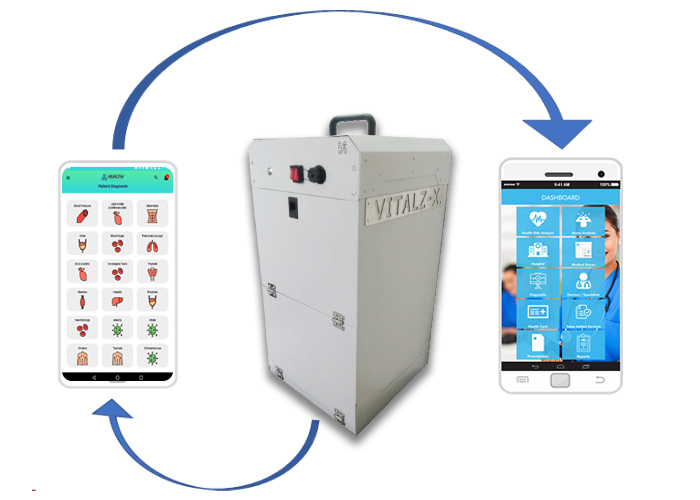
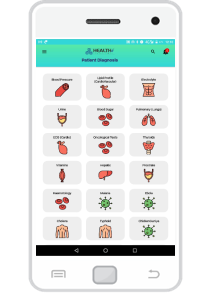
In order to achieve the above, CHART has developed a Cloud based SaaS (Software as a Service) Platform which includes a Web as well as Mobile Application called HealthE®. HealthE® allow Stakeholders to monitor the Prognosis, Diagnosis at real time and perform Management of different types of Patients, as well.
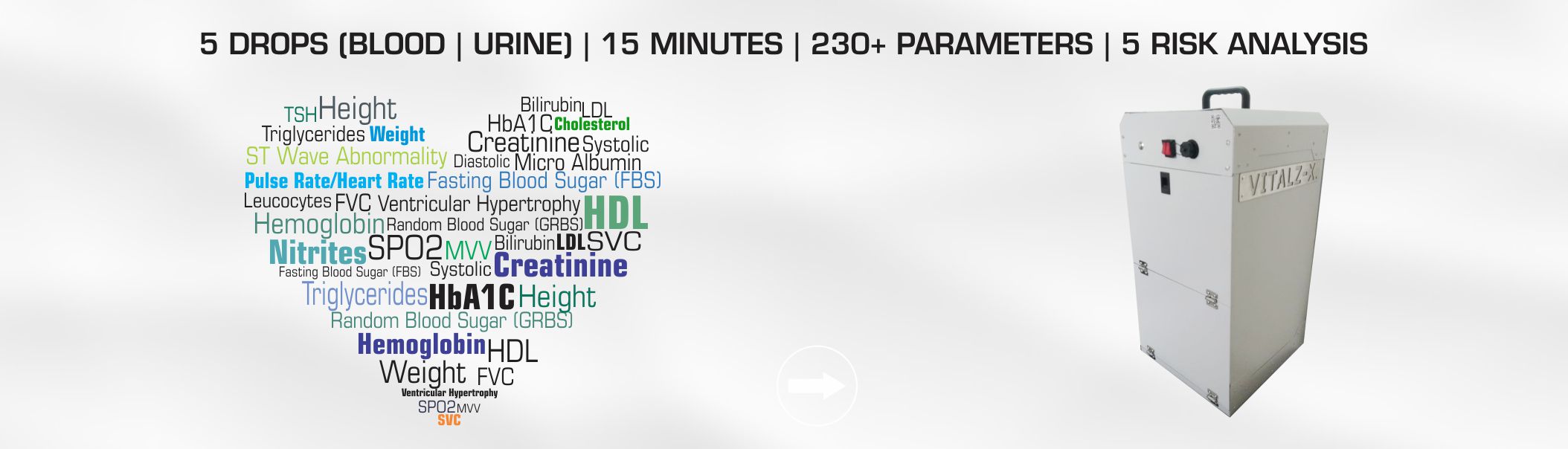
CHART has also developed a health screening device VitalZ-X® using IoT infrastructure. VitalZ-X is portable, it can perform Multiple (230 +) Diagnosis at the patient location and instantly deliver the result with minimal manual input. This allows immediate online Risk Analysis along with Reports & Alerts. Using this tool patients can be Monitored as well as Managed remotely. As per its product development strategy, CHART intends to keep on making multiple additions to existing version to make diagnosis easier and more accessible.